Key Takeaways
- Recent Advances in AI-Driven Gastrointestinal Endoscopy
- AI Applications in Endoscopy and GI Diagnostics: Transforming GI Care
- Enhancing Detection and Characterization of Polyps and Lesions
- Innovative Approaches in Capsule Endoscopy and Upper GI Screening
The integration of artificial intelligence into gastrointestinal medicine marks a significant advance in clinical practice. This technology directly addresses inherent limitations in human performance during endoscopic procedures.
Fatigue, stress, and varying experience levels among clinicians can impact diagnostic accuracy. Computational systems are designed to mitigate these factors. They bring a new level of consistency and precision to patient care.
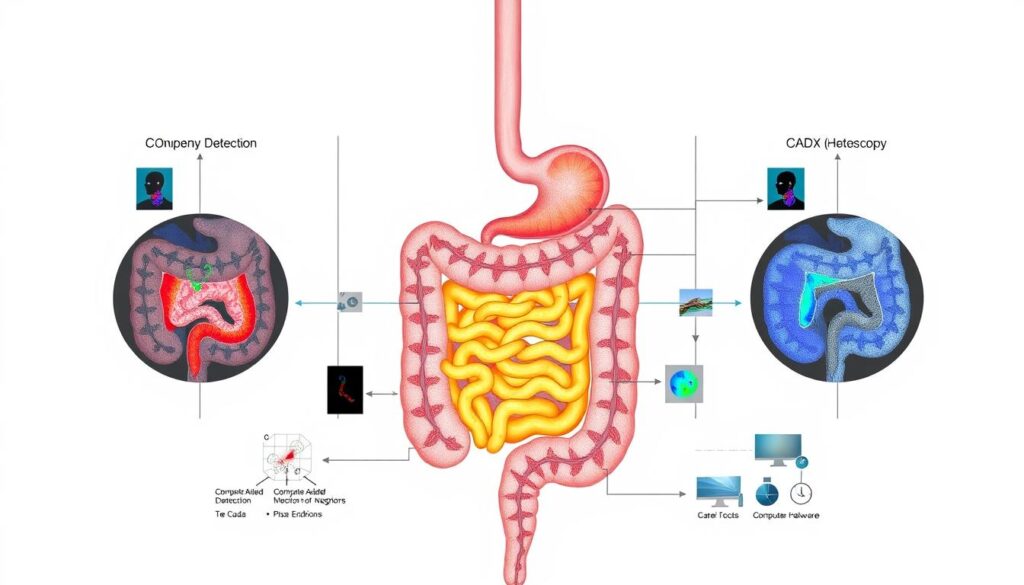
Two primary categories of systems have emerged for distinct clinical functions. Computer-assisted detection (CADe) focuses on lesion identification. Computer-assisted diagnosis (CADx) provides optical biopsy and lesion characterization capabilities.
These tools demonstrate measurable advantages. They reduce inter-operator variability and improve diagnostic accuracy. This facilitates prompt therapeutic decisions during procedures.
The future of this field depends on rigorous validation through multicenter trials. Standardization across diverse healthcare settings is essential. This ensures reliable performance, particularly for cancer screening programs outside expert centers.
Key Takeaways
- Artificial intelligence introduces a paradigm shift in gastroenterology by overcoming human performance limitations.
- Key benefits include improved diagnostic accuracy and reduced variability between different operators.
- Primary system categories are computer-assisted detection for finding lesions and computer-assisted diagnosis for analyzing them.
- Computer-aided quality assurance represents an advanced tier for comprehensive procedure monitoring.
- Video capsule endoscopy showcases a fully integrated system where algorithms operate with minimal human input.
- Successful clinical integration requires validation through rigorous, prospective multicenter trials.
- Regulatory frameworks are evolving to support the adoption of this technology in standard clinical practice.
Recent Advances in AI-Driven Gastrointestinal Endoscopy
Breakthroughs in deep learning methodologies are setting new benchmarks for performance in gastrointestinal imaging. These technological advances move beyond simple automation to provide robust decision support.
The progression of algorithms enables increasingly complex tasks during procedures.
Overview of AI Technologies in Endoscopy
Modern systems analyze both spatial and frequency domain features within images. This dual-analysis approach allows for highly accurate identification of subtle abnormalities.
Complex deep learning models are trained on extensive, annotated datasets. This training is fundamental to achieving high diagnostic metrics.
For instance, work by Hassan and Haque demonstrated sensitivities and specificities approaching 99% for bleeding detection. Similarly, Xiao and Meng achieved a 99% F1 score using a dataset of 10,000 capsule endoscopy images.
Clinical Successes and Breakthrough Studies
Substantial efficiency gains have been documented. Ding et al. reported software that reduces interpretation time for small-bowel capsule studies from 96.2 minutes to just 5.9 minutes.
This occurs while maintaining superior diagnostic accuracy compared to human experts.
A landmark meta-analysis of 28 trials provided level 1 evidence. It showed a 20% increase in adenoma detection rate and a 55% decrease in miss rate with computer-assisted colonoscopy.
This research synthesizes data from 23,861 participants, offering strong validation for these artificial intelligence tools. These studies confirm the tangible impact of this intelligence on clinical outcomes.
AI Applications in Endoscopy and GI Diagnostics: Transforming GI Care
Real-time computational assistance during gastrointestinal examinations offers significant enhancements to traditional diagnostic methods. These systems function as complementary tools that augment clinician capabilities rather than replace human expertise.
Role of CADe and CADx Systems
Computer-assisted detection systems serve as vigilant second observers during procedures. They utilize sophisticated machine learning models trained on extensive image databases to identify subtle mucosal abnormalities.
Computer-assisted diagnosis platforms extend beyond mere lesion detection. They provide real-time histological predictions through analysis of vascular patterns and surface architecture features.
Impact on Diagnostic Accuracy and Efficiency
Clinical studies demonstrate measurable improvements in diagnostic accuracy with these systems. The Wallace et al. study across four academic centers showed reduced adenoma miss rates with CADe implementation.
This technology enhances procedural performance by providing consistent, objective assessment. It reduces inter-operator variability that can affect diagnostic outcomes.
| Feature | CADe Systems | CADx Systems |
|---|---|---|
| Primary Function | Lesion detection | Lesion characterization |
| Clinical Application | Polyp identification | Optical biopsy |
| Impact on Workflow | Increased detection rates | Reduced pathology costs |
| Quality Metric | Reduced miss rates | Improved diagnosis accuracy |
Integration in Routine Clinical Practice
The World Endoscopy Organization supports CADe implementation for colorectal polyp detection. While short-term costs may increase, long-term cost-effectiveness is anticipated through cancer prevention.
Successful integration requires systematic evaluation of cost-effectiveness parameters. Standardized protocols ensure reliable performance across diverse healthcare settings, from tertiary centers to community facilities.
Enhancing Detection and Characterization of Polyps and Lesions
Modern diagnostic platforms leverage sophisticated algorithms to enhance visualization of colorectal polyps and lesions. These systems address specific challenges in mucosal abnormality identification that vary across practitioner experience levels.
AI-Enhanced Polyp Detection in Colonoscopy
European population studies by Wallace et al. demonstrated significant improvement in detecting diminutive or flat adenomas. This advancement proves particularly valuable for endoscopists with less experience in recognizing subtle lesion morphologies.
Computer-assisted detection devices show consistent adenoma detection rate increases across diverse populations. Benefits extend to proximal colon lesions and reduced polyp miss rates in large meta-analyses.
The technology maintains performance improvements across various healthcare settings and operator experience levels. Implementation considerations include modest withdrawal time increases and elevated non-neoplastic resection rates.
Optical Biopsy and Lesion Characterization Techniques
Hossain et al. documented characterization improvements with sensitivity reaching 90.9% compared to 48.1% without computational assistance. Negative predictive value achieved 95.8% with substantial accuracy improvements in both white-light and image-enhanced modalities.
Lesion characterization algorithms analyze vascular patterns, surface morphology, and color intensity. This enables precise differentiation between adenomatous and hyperplastic histology.
New computer-aided tools provide accurate polyp size measurement overcoming subjective estimation limitations. Fold-examination quality assessment systems offer objective metrics strongly correlating with expert scores and historical performance data.
Innovative Approaches in Capsule Endoscopy and Upper GI Screening
Video capsule endoscopy represents one of the earliest successful applications of computational systems in gastroenterology. These platforms incorporate sophisticated algorithms that analyze sequential images for clinical abnormalities.
Full AI Systems in Capsule Endoscopy
Multicenter prospective research by Spada et al. demonstrated significant advantages in diagnostic workflow. The study showed AI-assisted reading achieved 73.7% diagnostic yield compared to 62.4% with standard methods.
Reading time decreased dramatically from 33.7 minutes to just 3.8 minutes. Convolutional neural networks developed for bleeding detection achieved exceptional accuracy metrics of 98.5%.
Early Gastric Cancer Detection and Helicobacter pylori Diagnosis
The ENDOANGEL software system evaluated in 1,050 patients demonstrated remarkable performance. This tool reduced examination blind spots and detected early gastric cancers with 100% sensitivity.
Another diagnostic support system called Tango showed superior sensitivity compared to specialist endoscopists (84.7% versus 65.8%). These systems also demonstrate high accuracy in Helicobacter pylori diagnosis using endoscopic image data.
Pan-enteric capsule applications show strong agreement with standard evaluations for Crohn’s disease assessment. This extends computational utility beyond simple lesion identification to comprehensive disease characterization.
Challenges, Cost-Effectiveness, and Future Perspectives
Current challenges in the clinical adoption of intelligent diagnostic tools span technical, economic, and regulatory domains. Despite promising performance metrics in controlled study settings, real-world implementation faces substantial hurdles.
Evaluating Clinical Adoption and Workflow Integration
The absence of standardized evaluation frameworks represents a critical barrier. Parasa et al. highlighted the lack of uniform metrics for emerging technologies.
This necessitates comprehensive guidelines like the American Society for Gastrointestinal Endoscopy model cart. This framework addresses product specifications and post-market surveillance data.
Cost-effectiveness remains a primary concern without established reimbursement mechanisms. Long-term economic modeling suggests upfront costs may be offset by cancer treatment savings.
Addressing Limitations and Technical Drawbacks
Excessive false positive alerts disrupt endoscopic workflow and attention. Optimizing algorithm specificity and precision is essential for clinical utility.
Validation challenges arise from retrospective review methodologies. Prospective trials across diverse populations are needed for generalizability.
| Challenge Category | Specific Issues | Potential Solutions |
|---|---|---|
| Technical Limitations | False positive rates, workflow disruption | Algorithm refinement, specificity optimization |
| Economic Barriers | Lack of reimbursement, implementation costs | Long-term cost-benefit analysis, payment models |
| Validation Requirements | Retrospective study dominance | Prospective multicenter trials |
| Regulatory Considerations | Standardization needs, post-market monitoring | Comprehensive framework development |
Esophageal applications demonstrate particular promise. Multicenter research shows detection accuracy exceeding 90% for Barrett’s neoplasia.
Future validation must address real-world heterogeneity. This ensures reliable performance across diverse clinical settings.
Conclusion
Gastroenterology stands at the threshold of a new era defined by the synergistic partnership between clinician expertise and algorithmic precision. This collaboration represents a fundamental shift in diagnostic paradigms.
Substantial evidence from randomized controlled studies and systematic review confirms measurable improvements in lesion detection rates and diagnostic accuracy. These advancements enhance procedural quality across various endoscopy applications.
The future trajectory requires multicenter validation to ensure generalizability across diverse clinical settings. Successful implementation depends on standardized protocols and regulatory frameworks that support equitable access.
Artificial intelligence technologies have demonstrated their potential to transform gastrointestinal care delivery. Their integration promises enhanced patient outcomes through improved diagnosis and consistent performance metrics.
FAQ
What is the primary role of artificial intelligence in modern endoscopy?
The primary role is to augment the endoscopist’s capabilities through computer-aided detection (CADe) and diagnosis (CADx) systems. These tools enhance the identification and characterization of polyps, lesions, and other abnormalities, aiming to improve diagnostic precision and reduce miss rates during procedures.
How does AI improve the accuracy of polyp detection in colonoscopy?
AI algorithms analyze the video feed in real-time, highlighting suspicious regions with visual markers. This assistance has been shown in multiple studies to increase the adenoma detection rate (ADR), a key quality metric. The technology improves sensitivity for detecting subtle or flat lesions that might otherwise be overlooked.
What are the current limitations of AI integration in gastrointestinal diagnostics?
Current limitations include the need for extensive validation across diverse patient populations, integration into existing clinical workflows without disrupting efficiency, and addressing cost-effectiveness. Further research is required to ensure generalizability and to establish long-term outcomes data supporting widespread adoption.
Can AI be used for capsule endoscopy analysis?
Yes, artificial intelligence plays a significant role in capsule endoscopy by automating the review of the extensive video data captured. AI systems can flag images containing blood, ulcers, or lesions, substantially reducing reading time for physicians and potentially improving detection rates for small bowel diseases.
What future advancements are expected in AI-assisted endoscopy?
Future advancements are anticipated in the realm of predictive analytics and personalized medicine. This includes the development of systems that not only detect abnormalities but also predict disease progression or therapeutic response. Enhanced lesion characterization through “optical biopsy” techniques may also reduce the need for physical tissue sampling.