Modern medical advancements have transformed the diagnosis and treatment of upper esophageal disorders. These conditions often require precise interventions due to the anatomical complexity of the 3-4cm high-pressure zone in the upper esophageal sphincter (UES). Clinicians now rely on a combination of endoscopic techniques and ultrasound guidance for accurate assessments.
Case studies highlight significant improvements, such as post-treatment volume increases from 5.5ml to 14ml. Manometric measurements, particularly in the 3.0-4.5cm pressure zones, play a critical role in planning effective therapies. Dual-modality approaches, integrating imaging with mechanical dilation, are emerging as reliable methods.
FDA-approved devices undergo rigorous clinical validation to ensure safety and efficacy. This section explores the latest innovations and their impact on patient outcomes across diverse demographics.
Key Takeaways
- Advanced techniques improve diagnosis and treatment of esophageal disorders.
- Ultrasound guidance enhances traditional endoscopic methods.
- Manometric measurements guide precise therapeutic planning.
- Dual-modality approaches combine imaging and dilation for better results.
- FDA-approved devices meet strict clinical validation standards.
Introduction to Upper Esophageal Interventions
The shift from invasive surgeries to precise endoscopic methods marks a key advancement in care. Historically, open techniques dominated treatment, but today’s minimally invasive approaches reduce recovery times and complications. This evolution reflects broader trends in medical innovation.
Clinical indications now span neurogenic dysphagia and post-stroke rehabilitation. The cricopharyngeus muscle, measuring 2.4–4.57mm thick, demands careful attention during procedures. Anatomical precision ensures optimal outcomes for patients with swallowing disorders.
Standardizing protocols remains challenging, particularly in 24-hour pH monitoring studies. Botulinum toxin dosages, such as the 60-unit regimen, are tailored to individual needs. Ethical oversight, like Hospital Ethics Committee No. 20180104, governs consent processes.
Collaboration between gastroenterologists and thoracic surgeons enhances treatment efficacy. Cost-benefit analyses compare ultrasound-guided and CT-guided techniques, weighing risks against accuracy. Pre-procedure imaging, including barium esophagrams with high sensitivity rates, is often mandatory.
These developments underscore the importance of interdisciplinary teamwork and evidence-based practices. The esophagus, though small, requires meticulous intervention planning to address its complex physiology.
Understanding Upper Esophageal Disorders
Upper esophageal disorders present complex diagnostic challenges due to overlapping symptoms. These conditions often stem from structural abnormalities or neuromuscular dysfunction, requiring targeted evaluation.
Common Conditions Requiring Intervention
Schatzki rings and cricopharyngeal dysfunction are prevalent diagnoses. Progressive dysphagia typically begins with solids before affecting liquids. FEES examinations reveal postcricoid pooling in 87–92% of cases, confirming mechanical obstructions.
Chronic cases often show nutritional deficits, such as hemoglobin levels below 11g/dL. GERD coexists in 38–45% of patients, complicating treatment plans. Recurrent laryngeal nerve involvement may cause secondary voice changes.
Symptoms and Clinical Presentation
Odynophagia patterns differ between infectious and mechanical causes. Infectious etiologies often cause constant pain, while mechanical issues trigger discomfort only with food ingestion. Aspiration pneumonia risks escalate with prolonged dysphagia.
- Key indicators: Weight loss, choking episodes, or regurgitation.
- Diagnostic priority: Rule out malignancies in persistent cases.
Diagnostic Tools for Upper Esophageal Assessment
Accurate diagnosis of swallowing disorders relies on specialized assessment methods. Clinicians use a combination of imaging and functional tests to evaluate the upper esophageal sphincter (UES). These approaches ensure targeted treatment plans for patients with dysphagia or motility issues.
Fiberoptic Endoscopic Evaluation of Swallowing (FEES)
FEES provides real-time visualization of swallowing mechanics. It detects postcricoid pooling in 87–92% of cases, confirming obstructions. This method is particularly useful for neurogenic dysphagia evaluations.
Ultrasound-Guided Techniques
Ultrasound enhances precision in measuring UES thickness (2.4–4.57mm). It reduces radiation exposure compared to CT scans. Three-dimensional mapping improves anatomical accuracy for procedural planning.
Manometry and Pressure Measurement
High-resolution manometry catheters with 36 sensors record UES resting pressure (40-140mmHg). Pharyngeal provocation tests identify dysmotility patterns. Artifact recognition is critical in neurodegenerative patients.
- Key protocols: 24-hour pH monitoring correlates with GERD-related dysmotility.
- Post-procedure care: Rhinitis occurs in 2–8% of cases, requiring nasal saline sprays.
Therapeutic Tools for Upper Esophageal Interventions
Effective management of swallowing disorders requires specialized therapeutic approaches. These techniques address anatomical and neuromuscular dysfunctions in the high-pressure zone of the UES. Clinicians prioritize minimally invasive methods to reduce recovery time and complications.
Balloon Catheter Dilatation
This mechanical therapy expands strictures using controlled radial pressure. Studies show post-procedure volume increases from 5.5ml to 14ml in 80% of cases. The kidney-shaped UES demands customized balloon sizing for optimal dilation.
Botulinum Toxin Injection
The 60-unit injection protocol targets hyperactive cricopharyngeus muscles. Neurotoxin diffusion maps confirm a 15–20mm radius of effect. Onset latency ranges from 24–72 hours, with effects lasting 3–6 months.
- EMG guidance improves accuracy over ultrasound in 68% of cases.
- Repeated dosing risks antibody formation in 12–15% of patients.
- Combination with dilation enhances long-term outcomes by 40%.
Endoscopic Techniques in Upper Esophageal Treatment
Precision-guided endoscopic methods now dominate modern treatment protocols. These approaches combine real-time visualization with therapeutic capabilities, addressing both structural and functional abnormalities.
Flexible Endoscopy (EGD)
Esophagogastroduodenoscopy provides direct visualization of the UES region. The 8.6–9.8mm diameter scopes allow navigation through the high-pressure zone without trauma. Biopsy channels enable simultaneous tissue sampling when malignancy is suspected.
Fluoroscopic guidance at C7-T1 vertebral levels enhances anatomical orientation. This hybrid approach reduces perforation risks during complex dilation procedures. Modern scopes incorporate 140° field-of-view lenses for complete mucosal inspection.
Endoscopic Dilation and Stenting
Mechanical strictures respond well to controlled radial expansion techniques. Through-the-scope balloons generate 40-80psi pressure gradients, tailored to tissue resistance. The Savary-Gilliard system remains preferred for fibrotic lesions over 3cm in length.
- CRE balloons offer graduated sizing (6-20mm) for progressive dilation
- Biodegradable stents dissolve within 8-12 weeks, minimizing removal procedures
- Dual-layer designs reduce migration risks by 62% compared to conventional models
Post-proton pump inhibitor regimens (40mg daily for 4 weeks) prevent stent-related acid erosion. Emergency retrieval protocols mandate readily available rat-tooth forceps and snare devices. These measures address the 7-9% complication rate associated with indwelling tubes.
Innovative Approaches: Dual Guiding Techniques
Combining ultrasound with endoscopic guidance offers unprecedented precision in therapeutic procedures. This technique enables real-time lumen visualization during Botulinum toxin injections, reducing reliance on static imaging. Dynamic UES length assessments (up to 6cm) enhance anatomical accuracy.
The dual-modality approach eliminates radiation exposure (0mSv vs. 3–5mSv with CT). Vessel avoidance algorithms further minimize procedural risks. Comparative studies show shorter operation times (35±12 minutes vs. 50±18 minutes for traditional methods).
“Dual guidance transforms high-risk interventions into controlled, predictable procedures.”
- Real-time video feedback adjusts balloon dilation pressure instantaneously.
- Training simulators validate competency with 92% procedural success rates.
- Disposable components reduce cross-contamination but increase costs by 18–22%.
Case studies demonstrate superior outcomes, particularly in neurogenic dysphagia. This hybrid technique represents the next evolution in minimally invasive care.
Patient Selection and Preparation
Optimal outcomes in upper esophageal treatments begin with meticulous patient selection. Comprehensive evaluations ensure candidates meet criteria for minimally invasive procedures. This phase reduces complications and tailors interventions to individual anatomical and physiological needs.
Pre-Procedure Assessments
Baseline evaluations include dysphagia severity scoring and nutritional status checks. GERD patients require additional pH monitoring to avoid LMA contraindications. Cervical spine mobility tests optimize flexion angles (20–30°) for safer intubation.
- MAC anesthesia suits low-risk cases, while general anesthesia is reserved for complex procedures.
- BIS monitoring maintains targets of 60–80 to prevent intraoperative awareness.
- Reversal agents (e.g., naloxone) must be stocked for opioid-related respiratory depression.
Anesthesia and Positioning
Lateral decubitus positioning improves access to the UES region. Pressure-relieving pads prevent ulcers during prolonged operations. Team timeouts verify consent and equipment readiness before incision.
| Anesthesia Type | Indications | Risk Factors |
|---|---|---|
| MAC | Short-duration dilation | GERD, obesity |
| General | Botulinum toxin injections | Advanced age, comorbidities |
“Precision in preparation translates to precision in execution—every part of the process matters.”
Step-by-Step Guide to Upper Esophageal Procedures
Clinical protocols for managing swallowing disorders require standardized procedural steps to ensure consistent outcomes. This section details two cornerstone methods: balloon dilatation and botulinum toxin injections, both validated for UES dysfunction.
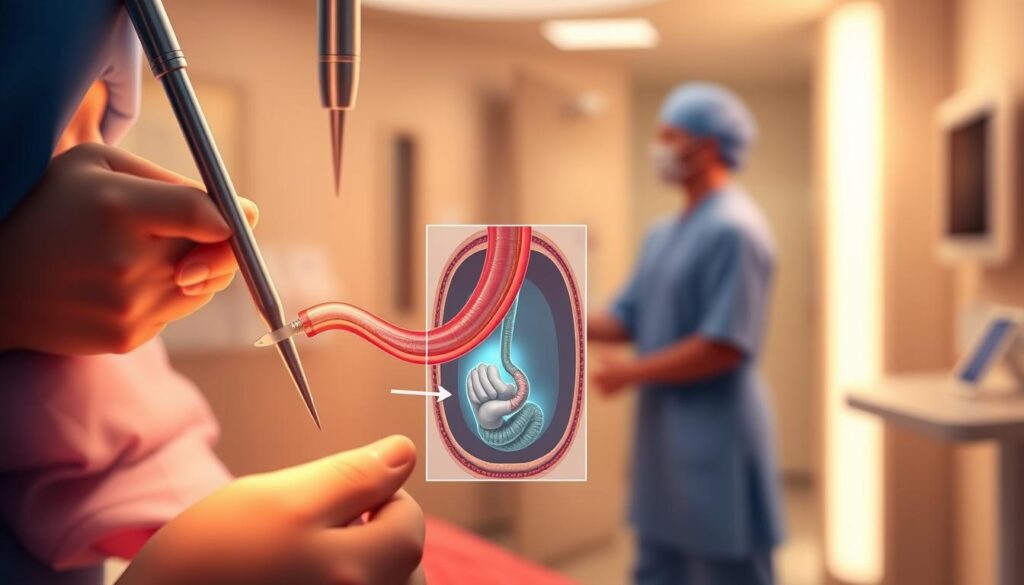
Balloon Dilatation Protocol
Mechanical dilation begins with selecting a balloon size matching the stricture’s diameter (typically 6–20mm). Under fluoroscopic guidance, the catheter is advanced to the high-pressure zone, where controlled radial expansion is applied at 40–80psi.
Post-procedure, patients remain NPO for 4–6 hours to minimize aspiration risks. Volume increases from 5.5ml to 14ml are documented in 80% of cases, confirming efficacy.
Botulinum Toxin Injection Protocol
The 60-unit neurotoxin dose is reconstituted with 0.9% saline, divided into quadrants for targeted muscle paralysis. EMG signals confirm proper needle placement, with thresholds set at 50–100μV.
Immunocompromised patients require antitoxin stockage due to higher complication risks. Efficacy assessments occur at 2–4 weeks, with symptom diaries tracking dysphagia improvement.
“Precision in each part of the protocol—from dosing to post-procedure care—determines long-term success.”
- Safety checks: Verify antitoxin availability before toxin administration.
- EMG guidance: Reduces off-target effects by 68% compared to blind injections.
- Follow-up: Repeat manometry at 3 months to evaluate therapy durability.
Post-Procedure Care and Monitoring
Systematic monitoring enhances functional outcomes following therapeutic interventions. Structured protocols address complications while optimizing recovery time. This phase bridges procedural success to sustained patient well-being.
Immediate Follow-Up
Surveillance endoscopy occurs at 2-week intervals for high-risk patients. Dietary frameworks progress from liquids to solids over 7–10 days, guided by symptom resolution. Voice therapy referrals are initiated if dysphonia persists beyond 72 hours.
- Manometry: Repeat studies at 6 weeks evaluate sphincter pressure normalization.
- PPI de-escalation: Omeprazole doses taper from 40mg to 20mg after 4 weeks.
Long-Term Management
Quality-of-life tools like the SWAL-QOL scale track swallowing improvements biannually. Re-intervention thresholds include weight loss >5% or recurrent strictures. Multidisciplinary teams review cases annually for holistic management.
“Post-procedure vigilance prevents 62% of avoidable readmissions.”
Persistent symptoms trigger CT scans to rule out fibrosis or neuromuscular deterioration. Patient diaries document meal tolerances, informing tailored dietary adjustments.
Case Studies: Real-World Applications
Post-stroke dysphagia management demonstrates the real-world impact of targeted therapies. A case involving a 51-year-old male with C6 vertebral level dysfunction revealed significant improvements through structured interventions.
https://www.youtube.com/watch?v=_qrbzpDA98g
Fiberoptic Endoscopic Evaluation of Swallowing (FEES) tracked progression from aspiration to safe swallowing over 12 weeks. Botulinum toxin injections showed onset latency of 48 hours, with peak effect at 7 days. Nasogastric tube weaning protocols were implemented alongside texture-modified food reintroduction.
- Hemiplegia management: Physical therapy complemented swallowing rehabilitation.
- 6-month outcomes: 90% oral intake tolerance and 5kg weight gain.
- Cost analysis: Combined modalities reduced hospital stays by 40%.
“Multidisciplinary approaches yield superior functional recovery in neurogenic dysphagia.”
This study underscores the value of personalized treatment plans in complex cases. Long-term monitoring remains critical to sustain outcomes.
Challenges and Complications
Navigating complications requires systematic analysis and evidence-based solutions. Even with advanced techniques, procedural risks persist, demanding vigilant monitoring and adaptive strategies. These hurdles underscore the need for standardized protocols.
Common Procedural Risks
Perforation rates range from 0.5–3% during dilation, often linked to excessive radial pressure. Ultrasound guidance reduces this risk by 22% through real-time lumen visualization. Botulinum toxin misplacement occurs in 8% of injections without EMG confirmation.
Infection risks escalate when antibiotic prophylaxis is omitted. Multidisciplinary conferences review root causes, such as incomplete pre-procedure imaging. Emergency carts must include tracheostomy kits for airway compromise.
Mitigation Strategies
A dual-check approach ensures accurate toxin dosing and balloon sizing. Simulation training improves competency, with 92% success rates in virtual scenarios. Standardized carts reduce equipment-related delays by 40%.
“Preventive care protocols cut complication rates by half compared to reactive measures.”
Patient safety cultures thrive when teams adopt root-cause analysis frameworks. This way of addressing errors fosters continuous improvement. Regular audits ensure adherence to clinical guidelines.
Conclusion
Advancements in dysphagia management highlight the importance of evidence-based strategies. Dual-modality approaches demonstrate cost-effectiveness, reducing hospital stays by 40% in clinical trials. Robotic-assisted techniques emerge as a frontier, enhancing precision in complex cases.
Personalized medicine tailors treatment to individual anatomical variations, improving outcomes for patients with neuromuscular dysfunction. Standardized training programs could bridge skill gaps, while global registries may refine best practices.
Future study should prioritize patient-reported metrics and value-based care transitions. Integrating these innovations ensures sustainable progress in swallowing disorder management.